Ongoing Medication Trials in Switzerland
Advancing drug therapies for neurodegenerative diseases
Neurodegenerative diseases, including Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis (ALS), pose a growing health challenge, with approximately 156,900 individuals affected by dementia in Switzerland alone (1). The therapeutic landscape for Alzheimer’s disease (AD) has undergone significant transformations, particularly with the 2021 approval of aducanumab, an anti-amyloid monoclonal antibody that ended a 17-year hiatus in new Alzheimer’s treatments (2).
Lecanemab and donanemab have emerged as recent additions to the treatment landscape for AD, both targeting aggregated amyloid-beta. Lecanemab received FDA approval in 2023, followed by donanemab’s approval in July 2024 (3,4). However, despite the excitement surrounding these therapies, lecanemab faced scrutiny when the European Medicines Agency (EMA) declined its approval, citing that the risks of severe side effects outweighed the anticipated benefits (5).
Global Landscape of Alzheimer’s Trials
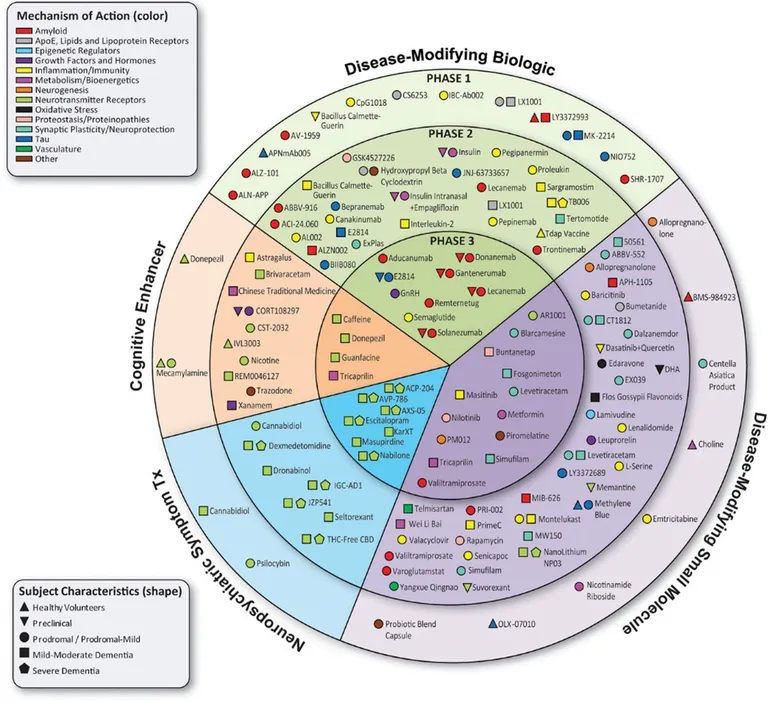
While these therapies are notable advancements, they represent just a fraction of the innovations in development. According to Cummings et al. (2024), there are currently 164 clinical trials evaluating 127 drugs for Alzheimer’s disease globally (6).
Figure 1 illustrates the current clinical trial landscape for AD, highlighting (6):
- 48 Phase 3 trials assessing 32 drugs
- 90 Phase 2 trials evaluating 81 drugs
- 26 Phase 1 trials testing 25 agents
Notably, 75% of these trials focus on disease-modifying therapies aimed at addressing the underlying biological mechanisms of Alzheimer’s, such as amyloid and tau pathology, neuroinflammation, oxidative stress, metabolism or synaptic plasticity. Among these, 24 agents target neuroinflammation, making up 19% of all therapeutic agents under development. In contrast, traditional targets like amyloid and tau account for 20 agents (16%) and 10 agents (8%), respectively (6).
The diverse range of targets in the Alzheimer’s drug development pipeline gives hope for effective treatments entering the market soon. However, it is important to note that the total development timeline for a potential Alzheimer’s therapy—from nonclinical studies to FDA review—averages approximately 13 years (6).
Current Status of Neurodegenerative Drug Trials in Switzerland
This global overview raises an important question: What is the status of ongoing drug therapy trials for neurodegenerative diseases in Switzerland? To address this, we investigated clinical trials registered on summarizes key medication studies currently underway in Switzerland, detailing the diverse therapeutic agents being tested, the specific diseases they target, the study phases and the sponsors behind these efforts (7).
Notably, many studies sponsored by major pharmaceutical companies, such as Biogen and Novo Nordisk, are often conducted as multicenter trials involving multiple participating sites. Currently, there are three ongoing trials in Switzerland that investigate the use of semaglutide for Alzheimer’s disease (note: these trials are not recruiting participants at this time). Semaglutide is a glucagon-like peptide-1 receptor agonist (GLP-1 RA) approved for the treatment of type 2 diabetes, but its potential applications in neurodegenerative diseases are being actively explored (8,9,10).
In addition, a Phase 3 trial conducted in Lausanne is examining the effects of gonadotropin-releasing hormone (GnRH) on cognitive dysfunction in individuals with Down syndrome. While GnRH is primarily recognized for its role in sex hormone production, it is also expressed in various brain regions, suggesting functions that extend beyond reproduction. Recent research indicates that GnRH may influence aging processes and lifespan control, making it an interesting target for further exploration (11).
Another study is underway at the Neurological Institute Konolfingen, focusing on the efficacy and safety of talineuren in patients with Parkinson’s disease. Talineuren is a liposomal formulation containing GM1 (monosialotetrahexosylganglioside), which has gained recognition as a promising neuroprotective agent (12,13).
In conclusion, the ongoing clinical trials in Switzerland reflect a proactive and innovative research environment dedicated to combating neurodegenerative diseases. With a focus on cutting-edge therapies, there is renewed hope for effective treatments that can significantly enhance the quality of life for those affected by these challenging conditions.